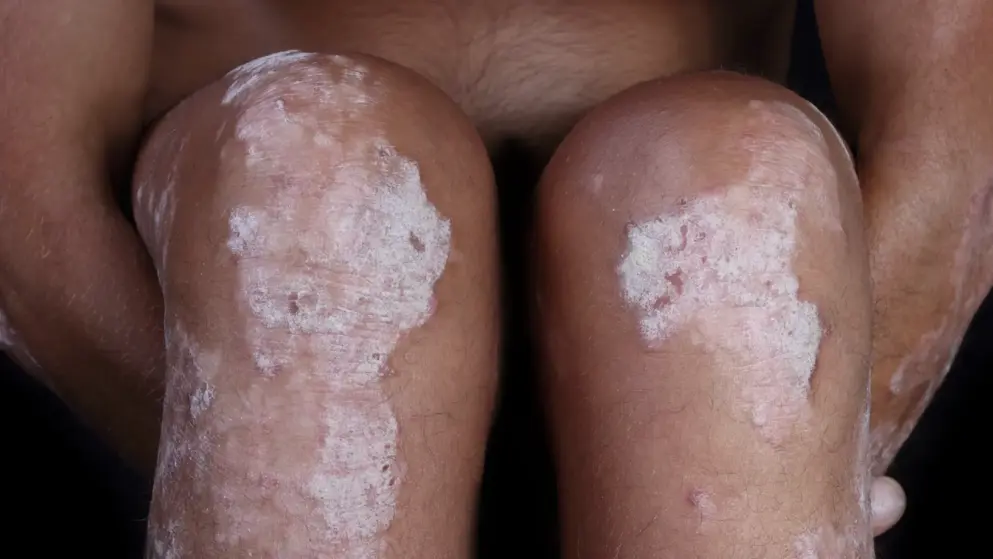
MHRA (UK) approves Sotyktu to treat moderate-to-severe plaque psoriasis.- BMS.
Bristol Myers Squibb's Sotyktu (deucravacitinib) has been authorised for use in the UK to treat adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
The decision from the Medicines and Healthcare products Regulatory Agency (MHRA) was supported by positive results from two phase III trials, in which once-daily Sotyktu was associated with superior improvements in skin clearance, symptom burden and quality of life measures compared to both placebo and twice-daily Otezla (apremilast).
It is estimated that up to 1.8 million people in the UK are affected by some form of psoriasis, a life-long inflammatory condition that typically affects the skin, nails and joints. Up to 90% of patients have plaque psoriasis, characterised by distinct round or oval plaques typically covered by silvery-white scales.