Publication of Psoriasis Patient Data demonstrating need for rapid symptom improvement
Novan, Inc. and MC2 Therapeutics announced that results of a recently conducted survey to assess psoriasis patients’ topical treatment experience, expectations , and preferences have been published in the Journal of Drugs in Dermatology (JDD)
The survey was conducted by the National Psoriasis Foundation and was sponsored by the Company. Most of the participants self-reported having moderate psoriasis (83.9%); most were using systemic therapies (73.5%) and employing topical medications at least once per week (84%).
“The most effective medications are the ones that patients actually use. Understanding what factors impact adherence can help guide physicians in our choice of topical psoriasis treatments.” commented Steven R. Feldman, M.D., Ph.D., Professor of Dermatology, Wake Forest University School of Medicine. “Findings from this survey demonstrated that psoriasis patients expect to see rapid improvement of their symptoms, or they report they will discontinue topical treatment. Selecting medications that work quickly and fit their formulation and vehicle preferences may improve adherence and successful treatment outcomes.”
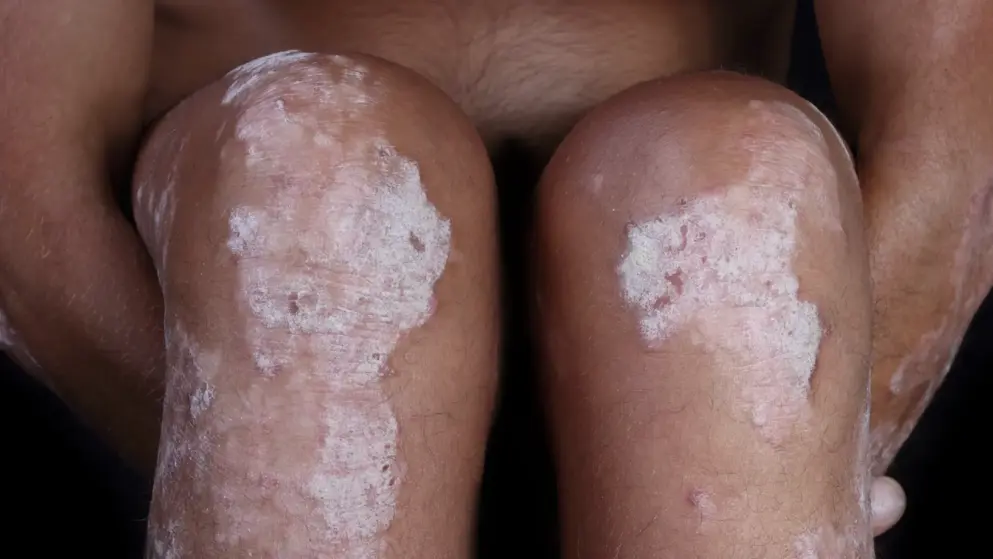
Key findings from the published results of the conducted survey include: i. More than 90% of respondents stated they suffered from itch frequently or always, with the majority of them rating this as moderate, severe or very severe (93%), and most often affecting the scalp, elbows and arms, and trunk. ii. Nearly 80% of participants in the survey said they would discontinue a topical treatment if they did not notice an improvement in their symptoms in two weeks or less. Only 5% of patients surveyed would be willing to persevere for more than a month without seeing results. iii 40% of patients stated they would call a different dermatologist if a treatment they were prescribed caused an unwanted reaction. iv. 75% of participants stated they would only use a medication for a week if they did not like a formulation that was prescribed for them. The attributes rated most important were application feel (55.2%), non-staining (49.9%), quick absorption (46.7%) and non-sticky texture (39.7%).
Wynzora (calcipotriene and betamethasone dipropionate) Cream is a once-daily aqueous topical medication that combines the benefits of a high potency steroid plus Vitamin D for the treatment of plaque psoriasis. It demonstrates rapid onset of action with results seen in efficacy, scale and itch as early as 1 week and continued improvement at 8 weeks. Under a Promotion and Collaboration agreement between Novan and MC2 Therapeutics Novan has exclusive detailing and distribution rights and the right to jointly with MC2 Therapeutics to engage in promotional, regulatory and certain commercialization activities for Wynzora Cream in the United States. MC2 Therapeutics granted Novan an exclusive right and license under MC2 Therapeutics’ intellectual property rights to sell, or detail (as defined in the agreement), and engage in certain commercialization activities for Wynzora Cream, in the United States.
Wynzora Cream is a fixed dose combination of calcipotriene and betamethasone dipropionate indicated for the topical treatment of plaque psoriasis in adults. Betamethasone dipropionate, a high-potency corticosteroid, has anti-inflammatory effects resulting in decreased expression of key TNF-alpha, IL-17A/F and IL-23 cytokines. Calcipotriene, a vitamin-D analog, adds to the anti-inflammatory effect of the corticosteroid by mediating psoriasis specific immune-modulating activity, stabilizes the Th2- and T-reg cell activity and inhibits epidermal hyperproliferation.
Wynzora Cream uses PAD Technology which uniquely enables calcipotriene and betamethasone dipropionate to be combined in a stable, convenient-to-use aqueous formulation. Wynzora Cream was designed to provide one product for high efficacy, a favorable safety profile and patient satisfaction. While their pharmacologic and clinical effects are known, the exact mechanisms of actions of betamethasone dipropionate and calcipotriene, and Wynzora Cream, in plaque psoriasis are unknown.
See- . Curcio A. et al. J Drugs Dermatol. 2023;22(4): doi:10.36849/JDD.7372.