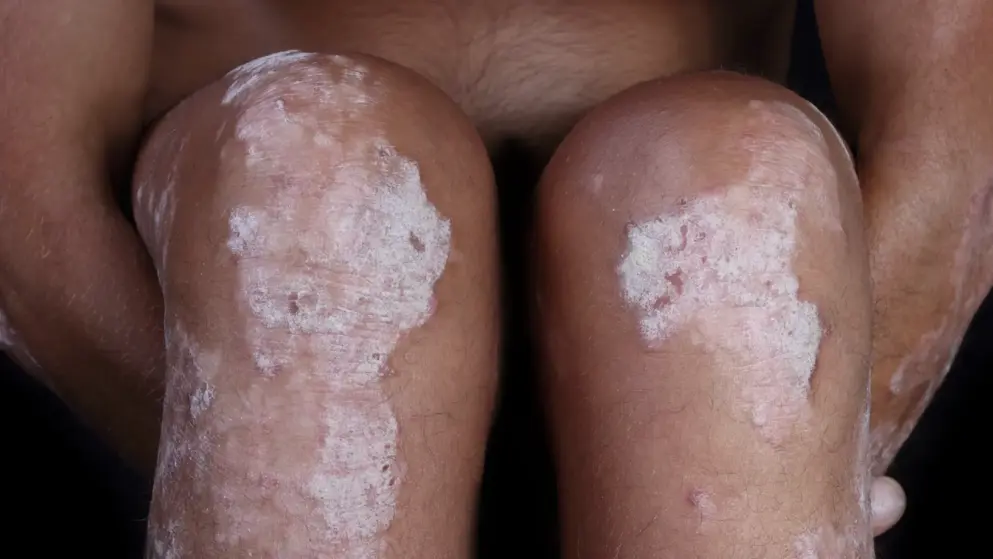
Health Canada approves Zoryve to treat psoriasis. -Arcutis Biotherapeutics Inc.
Arcutis Biotherapeutics, Inc. announced that its next-generation topical PDE4 inhibitor therapy for plaque psoriasis, Zoryve (roflumilast) cream 0.3%, has received regulatory approval from Health Canada. In Canada, Zorvye is indicated for topical treatment of plaque psoriasis, including treatment of psoriasis in the intertriginous areas, in individuals 12 years of age and older.
“The successful approval of Zoryve in Canada is a testament to Arcutis’ continued commitment to provide therapies that address the most persistent challenges for individuals with immune-mediated skin diseases. We are proud to bring this important new steroid-free cream to Canada, where there is a need for safe and effective topical treatment for plaque psoriasis,” said Frank Watanabe, President and CEO of Arcutis. “We deeply value our ongoing partnership with Canadian dermatologists, who played a central role in the development of Zoryve, providing approximately one-third of all clinical trials participants, and we look forward to continuing to advance our robust pipeline in both Canada and the United States.”
“Topical agents are the mainstay of treatment for the majority of individuals with plaque psoriasis, and until now there have been limited options suitable for chronic use. Zoryve demonstrates rapid clearance of plaques anywhere on the body, including harder to treat areas such as the elbows and knees, but is also effective in the sensitive intertriginous areas like under the breasts, in the groin, or under the arms,” said Dr. Melinda Gooderham, Assistant Professor, Queen’s University, Medical Director at the SKiN Centre for Dermatology and Principal Investigator for the SKiN Research Centre in Peterborough, Ontario.