Understanding alopecia areata
The epidemiology of alopecia areata
Patient advocate Lynn Wilks (UK) describes her early experiences with alopecia areata and how these first signs and symptoms affected her. View transcript.
Clinical variants of alopecia areata
Alopecia areata (AA) is a common form of non-scarring hair loss, which can involve total or near-total hair loss on the scalp and other haired body areas. There are several recognized clinical variants of AA (Figure 1).
Figure 1. Clinical variants of AA. (A) Circumscribed, “patchy” hair loss. (B) Alopecia totalis, 100% scalp hair loss. (C) Hair loss of the occipital area (ophiasis AA). (D) Partial loss of eyebrows and eyelashes. (E) Patchy hair loss of the beard. All photos from DermNet reused under Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 New Zealand (CC BY-NC-ND 3.0 NZ). AA, alopecia areata.
The epidemiology of alopecia areata
The younger the age of onset, the greater the lifetime likelihood of severe AA
AA affects people of all ages, including children and adolescents.1 People with skin of color are disproportionately affected by AA, with those of African and Asian descent experiencing higher rates when compared with White populations.2 People of Asian descent are three times more likely to experience AA when compared with other populations.2
AA has an estimated lifetime prevalence of 0.10% in the general population – 0.12% in adults and 0.03% in children. AA is most prevalent in Asia.3 Further data from low- and middle-income countries is required to fully understand the global burden of AA.3
Of people with alopecic patches persisting after 1 year, 30% develop alopecia totalis (AT), and 15% develop alopecia universalis (AU).4 Prevalence rates of the more severe forms are 0.08–0.15% for alopecia ophiasis and AT, and 0.01–0.07% for AU.5-8 AA follows a variable relapsing and remitting course that is difficult to predict.9
Men and women are affected equally. Male patients tend to be diagnosed at a younger median age (31 years) than female patients (36 years) and have a poorer prognosis for hair regrowth than females.10
The younger the age of onset, the greater the lifetime likelihood of severe AA (AT and AU). Severe AA often occurs alongside other conditions, such as atopic dermatitis, eczema, asthma, vitiligo, psoriasis, lichen planus, thyroid conditions, vitamin D deficiency, and anemia.11
References
- Lintzeri, 2022. Alopecia areata – Current understanding and management. https://www.doi.org/10.1111/ddg.14689
- Harries, 2022. The epidemiology of alopecia areata: A population-based cohort study in UK primary care. https://www.doi.org/10.1111/bjd.20628
- Jeon, 2024. Global, regional and national epidemiology of alopecia areata: A systematic review and modelling study. https://www.doi.org/10.1093/bjd/ljae058
- Cranwell, 2019. Treatment of alopecia areata: An Australian expert consensus statement. https://www.doi.org/10.1111/ajd.12941
- Pratt, 2017. Alopecia areata. https://www.doi.org/10.1038/nrdp.2017.11
- Lee, 2020. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. https://www.doi.org/10.1016/j.jaad.2019.08.032
- Fukuyama, 2022. Alopecia areata: Current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. https://www.doi.org/10.1111/1346-8138.16207
- Vu, 2022. EPH57 epidemiology of alopecia areata across global regions: A systematic literature review. https://www.doi.org/10.1016/j.jval.2022.09.979
- Zhang and Nie, 2022. Prediction of the risk of alopecia areata progressing to alopecia totalis and alopecia universalis: Biomarker development with bioinformatics analysis and machine learning. https://www.doi.org/10.1159/000515764
- Mirzoyev, 2014. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. https://www.doi.org/10.1038/jid.2013.464
- Lizarondo, 2021. Determination of serum 25-hydroxyvitamin D levels in patients with alopecia areata and their comparison with levels in healthy controls: A cross-sectional study. https://www.doi.org/10.1016/j.jdin.2021.07.008
Understanding the pathophysiology of alopecia areata
Although the exact pathophysiology of hair loss in alopecia areata (AA) is unknown, it is widely accepted as a complex T-cell–mediated autoimmune process, where hair follicles (HF) prematurely skip from the growing anagen phase to the resting telogen phase, resulting in hair loss, as illustrated in Figure 1.1
Figure 1. The hair cycle in alopecia areata.2,3
Damage to the HF can result from an inherited susceptibility, polygenic oxidative stress, infection, or inflammation. These factors can lead to a loss of HF immune privilege (HF-IP) and an autoimmune attack. A local surge of interferon gamma (IFNγ) activates the cytotoxic CD8+ NKG2D+ T cells, which destroy the exposed HF autoantigens.1
The healthy HF maintains its immune privilege by suppressing major histocompatibility complex (MHC) class I and β2 microglobulin. This then stimulates natural killer (NK) cells, producing immunosuppressants, stimulating immunoinhibitory signals such as CD200, and suppressing intrafollicular antigen-presenting cells (APCs) and perifollicular NK cell and mast cell functions, owing to increased levels of macrophage migration inhibitory factor (MIF).
Vasoactive intestinal peptide (VIP), released by perifollicular sensory nerve fibers, contributes to the immune privilege.6
Late anagen HFs in people with AA show infiltrates of APCs, CD4+, and CD8+ T cells, and an abnormal increase in MHC class I and II molecules. CD8+ T cells penetrate the HF root sheaths.6
As AA activity subsides, the HFs progress further into the anagen phase. Proximal weakness of the hair shaft leads to the cardinal sign of “exclamation point” hair.1,2
Genetic predisposition to alopecia areata
AA is likely to have a hereditary predisposition; 10–20% of people with AA have positive family histories, compared with 1.7% of control participants.4-6
Genetic susceptibility to AA has been identified in 14 gene loci in the HLA (human leukocyte antigen) class I region that signal for HF maturation. In addition to autoimmunity, people with AA are susceptible to abnormal keratinization of the hair shaft.4-6
Genetic studies and genome-wide association studies show that CD8+ NKG2D+ T cells are the major effectors of AA disease pathogenesis.2 Polygenic susceptibility is likely responsible for AA initiation and variation in clinical subtypes.2,7
Environmental triggers of alopecia areata
Mental and physical stress, vaccinations, febrile illnesses, and drugs are often linked with the onset and/or worsening of AA symptoms. Viruses – such as Epstein–Barr virus (EBV), hepatitis B and C virus, the swine flu virus, and SARS CoV-2 – have been implicated in the autoimmune reaction associated with AA.2,3,8,9
Mediators induced by mental stress, such as corticotropin-releasing hormone (CRH), substance P, and nerve growth factor, act through the hypothalamic–pituitary axis. Prolonged stress causes a cytokine imbalance by activating tumor necrosis factor-alpha (TNFα), IFNγ, and T-helper cell type 1, damaging epithelial and mesenchymal cells, and stopping hair growth.10-12
Collapse of immune privilege in alopecia areata
Healthy HFs are immune to attack as they have no lymphatics, copious extracellular matrix, and separated antigens, while NK cells are suppressed.13 These barriers, combined with the downregulation of MHC class I and II molecules, maintain HF-IP. Overexpression of macrophage MIF, an NK cell inhibitor, prevents a type of T lymphocyte – CD56+/NKG2D+ NK cells – from invading the HF.13
Macrophages in the HF root sheath cells that express IFNγ stay repressed under the influence of immune guardians, such as transforming growth factor (TGF)-β, alpha-melanocyte-stimulating hormone (α-MSH), interleukin(IL)-10, macrophage MIF, and somatostatin.2
Environmental triggers cause abnormal MHC class I and II expression, leading to inflammatory cell infiltrates. The cytotoxic CD56+/NKG2D+ NK cells start hypersecreting IFNγ to initiate a collapse of the HF-IP.8,14
HF autoantigens responsible for AA autoimmunity (tyrosinase-related protein, gp100, trichohyalin, melanoma antigen, and retinol-binding protein) are immune-recognized after the collapse of HF-IP. Lastly, the vasoactive intestinal peptide receptors (VIPR1 and VIPR2) in the HF epithelium are downregulated with defective signaling in the hair bulbs of people with AA.2,10-12
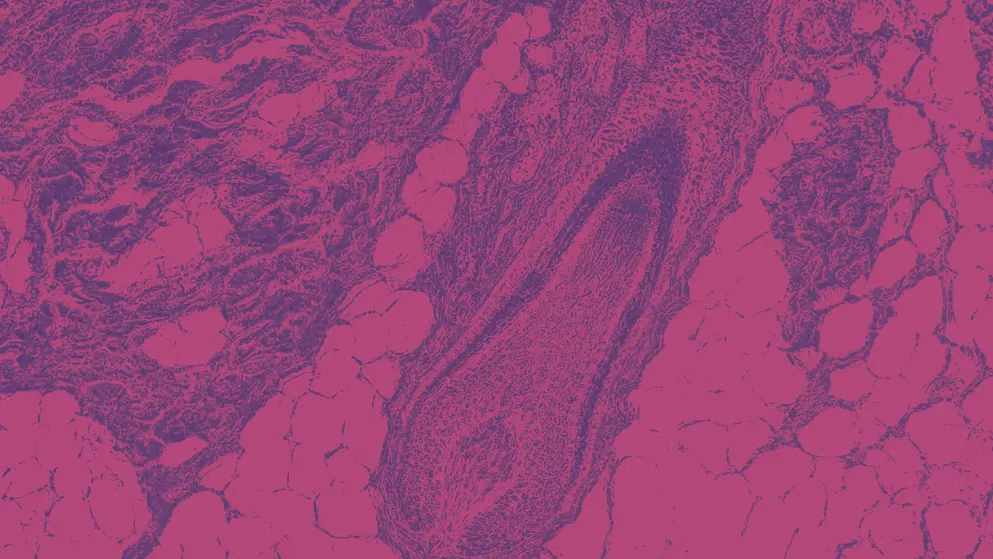
A dense perifollicular inflammatory cell infiltration, known as a “swarm of bees,” and damaged HFs are observed in the acute phase of AA. In the chronic phase of AA, the cell infiltration subsides, and there is a prolonged telogen phase (Figure 2).11
Figure 2. Histopathology in acute and chronic alopecia areata.3,11 Acute alopecia areata is marked by a dense perifollicular inflammatory infiltrate and damaged hair follicles. In the chronic phase, inflammatory cell infiltrate decreases, and the catagen or telogen phase is prolonged.
Interferon gamma and the JAK–STAT pathway
A JAK–STAT-dependent type 1 cytokine loop can lead to the prolongation of AA and chronic disease14,15
Interferon gamma as mediator of type 1 cytokines in alopecia areata
The primary mediator of type 1 cytokines in AA is IFNγ. When IFNγ binds to receptors in the HF epithelium, C-X-C motif chemokine receptor 3 (CXCR3) and its ligands CXCL9, CXCL10, and CXCL11, MHC class I, and MICA (MHC class I polypeptide-related sequence A) are induced via the JAK–STAT pathway (Figure 3).
Figure 3. Initial autoimmune mechanisms in alopecia areata.14 Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0). AA, alopecia areata; CXCL, chemokine (C-X-C motif) ligand; CXCR3, C-X-C motif chemokine receptor 3; GZMB, granzyme B; IFN, interferon; IL, interleukin; JAK, Janus kinase; MHC, major histocompatibility complex; MICA, major histocompatibility complex class I chain-related gene A; NK, natural killer; PDC, plasmacytoid dendritic cell; Th cells, T-helper cells; TNF, tumor necrosis factor; Treg cells, regulatory T cells; ULBP, UL16-binding protein.
This JAK–STAT-dependent type 1 cytokine loop leads to the prolongation of AA and chronic disease.14,15
Role of type 2 cytokines in alopecia areata
Although predominantly a type 1 inflammation, people with AA also show type 2-related biomarkers, such as IL-4, IL-13, CCL18, and thymic stromal lymphopoietin (TSLP) in scalp lesions, and raised levels of IL-4, IL-5, IL-6, CCL17, immunoglobulin E, and eosinophilia in the serum.14,15 A third axis of pro-inflammatory T-helper-17 (Th17)–related cytokines IL-17 and IL-22 underpins the more severe and refractory forms of AA.16,17
Future research
Researchers have recently uncovered links between AA and cardiovascular disease, obesity, and even the microbiota.
Serum levels of cardiovascular biomarkers, such as cardiac troponin I (cTnI) and N-terminal pro-B type natriuretic peptide (NT-proBNP), are elevated in people with AA who are free of cardiovascular disease and its risk factors. This suggests that the inflammatory nature of AA could result in subtle myocardial infarction.18
Obesity and metabolic syndrome often exist alongside AA, potentially indicating a common pathogenic pathway.19 In a 2021 study, serum levels of adiponectin decreased as AA severity increased, supporting shared mechanisms between AA and obesity.19
The role of the microbiota in the pathogenesis of AA is an emerging area of research, with some success reported with fecal microbiota transplants.20 This aspect of AA requires further research to be fully understood and may yet yield future treatment targets for AA.21
References
- Simakou, 2019. Alopecia areata: A multifactorial autoimmune condition. https://www.doi.org/10.1016/j.jaut.2018.12.001
- Pratt, 2017. Alopecia areata. https://www.doi.org/10.1038/nrdp.2017.11
- Gilhar, 2012. Alopecia areata. https://www.doi.org/10.1056/NEJMra1103442
- Jackow, 1998. Alopecia areata and cytomegalovirus infection in twins: genes versus environment? https://www.doi.org/10.1016/s0190-9622(98)70499-2
- Rodriguez, 2010. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. https://www.doi.org/10.1016/j.jaad.2009.02.006
- Betz, 2015. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. https://www.doi.org/10.1038/ncomms6966
- Oka, 2020. Alopecia areata susceptibility variant in MHC region impacts expressions of genes contributing to hair keratinization and is involved in hair loss. https://www.doi.org/10.1016/j.ebiom.2020.102810
- Mcelwee, 2013. What causes alopecia areata? https://www.doi.org/10.1111/exd.12209
- Christensen and Jafferany, 2022. Association between alopecia areata and COVID-19: A systematic review. https://www.doi.org/10.1016/j.jdin.2022.02.002
- Tzur Bitan, 2022. The association between alopecia areata and anxiety, depression, schizophrenia, and bipolar disorder: a population-based study. https://www.doi.org/10.1007/s00403-021-02247-6
- Fukuyama, 2022. Alopecia areata: Current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. https://www.doi.org/10.1111/1346-8138.16207
- Peters, 2007. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. https://www.doi.org/10.2353/ajpath.2007.061206
- Ito, 2008. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. https://www.doi.org/10.1038/sj.jid.5701183
- Suchonwanit, 2021. Alopecia areata: An autoimmune disease of multiple players. https://www.doi.org/10.2147/ITT.S266409
- Lensing and Jabbari, 2022. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. https://www.doi.org/10.3389/fimmu.2022.955035
- Dai, 2021. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. https://www.doi.org/10.1172/jci.insight.142205
- Crowley, 2019. The use of Janus kinase inhibitors in alopecia areata: A review of the literature. https://www.doi.org/10.1177/1203475418824079
- El-Sayed Mahmoud Marie, 2021. Evaluation of serum cardiac troponin I and N-terminal pro-B-type natriuretic peptide levels in patients with alopecia areata. https://www.doi.org/10.1111/ced.14425
- Stochmal, 2021. Adiponectin as a novel biomarker of disease severity in alopecia areata. https://www.doi.org/10.1038/s41598-021-92853-1
- Paus, 2020. The evolving pathogenesis of alopecia areata: Major open questions. https://www.doi.org/10.1016/j.jisp.2020.04.002
- Sánchez-Pellicer, 2022. How our microbiome influences the pathogenesis of alopecia areata. https://www.doi.org/10.3390/genes13101860
The impacts of alopecia areata
The burden of alopecia areata
Disease burden is commonly evaluated by disability-adjusted life years (DALY), which combine years lost to morbidity and mortality. Globally, the mean DALY in alopecia areata (AA) were approximately 19.4 years across 21 geographic regions – and remained unchanged from 1990–2010.1-5
What are the impacts of AA on individuals? Patient advocate Lynn Wilks shares her lived experiences with AA, detailing its impact on her daily life, and dermatologists Brett King and Rodney Sinclair highlight the unmet needs of people with AA and the emotional significance of hair.
Quality of life
Over half of people with AA report poor health-related quality of life (HRQoL). Risk factors for low HRQoL are age between 20 and 50 years, female sex, lightening of skin color, hair loss greater than 25%, family stress, and job change. People with extensive AA report more adverse psychological consequences than those with less severe AA.3,5
Notably, AA ranks higher in disrupting HRQoL than melanoma, non-melanoma skin cancer, and psoriasis.1,6,7
Psychiatric comorbidity
Patient advocate Lynn Wilks describes the evolving relationship between AA and her sense of self. View transcript.
There is a 66–74% lifetime prevalence of psychiatric disorders in people with AA, with a 39–62% prevalence of anxiety and a 38–39% lifetime prevalence of depression.3
There is an increased risk of depression in people with AA younger than 20 years and an increased risk of anxiety in those aged 40–59 years.3
Hair loss can negatively affect body image, leading to sleep problems and sometimes adolescent suicides.8 Almost one-third of people with AA will lose their existing partners because of the other person’s inability to cope with their changed appearance.9 The absence of effective therapies can contribute to feelings of hopelessness and persistent anxiety about potential future hair loss.1,10
Comorbid medical conditions
Comorbid medical conditions can adversely affect disability levels, quality of life, and psychiatric comorbidity. Clinicians caring for people with AA should consider assessing for comorbid conditions because:3
- People with a family history of vitiligo are more likely to develop extensive or severe AA
- Atopic dermatitis was reported in 15.6% of adults with AA and in 39.5% of children with AA
- Prevalence of thyroid peroxidase antibodies in people with AA is 17.7% – approximately double that of the general population, with a female-to-male predominance of 6.7:1
References
- Liu, 2016. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): A systematic review. https://www.doi.org/10.1016/j.jaad.2016.04.035
- Karimkhani, 2017. Global skin disease morbidity and mortality an update from the global burden of disease study 2013. https://www.doi.org/10.1001/jamadermatol.2016.5538
- Harries, 2022. The epidemiology of alopecia areata: A population-based cohort study in UK primary care. https://www.doi.org/10.1111/bjd.20628
- Lintzeri, 2022. Alopecia areata – Current understanding and management. https://www.doi.org/10.1111/ddg.14689
- Villasante Fricke and Miteva, 2015. Epidemiology and burden of alopecia areata: A systematic review. https://www.doi.org/10.2147/ccid.S53985
- Dainichi and Kabashima, 2017. Alopecia areata: What's new in epidemiology, pathogenesis, diagnosis, and therapeutic options? https://www.doi.org/10.1016/j.jdermsci.2016.10.004
- Korta, 2018. Alopecia areata is a medical disease. https://www.doi.org/10.1016/j.jaad.2017.09.011
- Marahatta, 2020. Psychological impact of alopecia areata. https://doi.org/10.1155/2020/8879343
- Sinclair, 2014. Alopecia areata and suicide of children. https://doi.org/10.5694/mja13.10895
- Pratt, 2017. Alopecia areata. https://www.doi.org/10.1038/nrdp.2017.11
Developed by EPG Health. This content has been developed independently of the sponsor, Pfizer, who has reviewed the content only for scientific accuracy. EPG Health received funding from the sponsor in order to help provide healthcare professionals with access to the highest quality medical and scientific information, education and associated relevant content.







